Heat Theory and Global Warming
This text has been officially registered.
No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without written permission of the publisher.
Copyright © 2019 Maurizio Vignati
This article is published in the issue 158 2021 of the magazine “Infinite Energy”. The entire item can be purchased by accessing the following address:
https://www.infinite-energy.com/store/index.php?main_page=product_info&cPath=11&products_id=576
HEAT THEORY AND GLOBAL WARMING
Chapter 1 – Summary
The measures that the various Nations have put in place so far to counteract the global warming, are based on current scientific knowledge and it is a common opinion that measures of greater effectiveness cannot exist, since science is considered infallible.
In reality, scientists continually improve their knowledge on the composition and behaviour of Nature following the Scientific Method, according to which any scientific theory has a temporary character.
A theory concerning a certain natural phenomenon arises from experimental observation, but the Scientific Community remains in constant expectation of any other experiments that give different results, or that are in contradiction with the theory itself; it is sufficient that only one of them should be verified, provided that it is duly documented, so that scientists begin to consider this theory incorrect.
In this regard, the phrase contained in a letter which Albert Einstein (1879-1955) wrote on 4 December 1926 to the physicist Max Born (1882-1970) is famous:
“No amount of experiments can prove that I’m right; a single experiment will prove that I was wrong.”.
In summary, experience can never serve to confirm a scientific theory, but can only demolish it; this is the rigor of the Scientific Method.
What is nowadays considered scientific and unassailable, perhaps one day will make scientists smile; the history of science suggests this: all those theories on the composition and behaviour of Nature that were once considered valid are now considered wrong.
That said, in this document it is argued that there is a different way from all those considered so far to combat the global warming. However, mankind cannot put this system into practice, unless it first acknowledges the complete and total incorrectness of the version of the Second Principle of Thermodynamics currently shared by most of the Scientific Community.
Below is a summary of the analysis I conducted on the incorrectness of the aforementioned Principle – a path that began during the degree course in Physics, and continued for over fifty years until today.
The generic reader should not be afraid that the scientific subject discussed here will prevent him from understanding the contents of this memoir, since there are no equations and the text is designed in such a way as to be understood by anyone with a normal ability to use Logic.
Chapter 2 – Introduction
At first glance, the idea of countering global warming by dealing with Thermodynamics may seem an oddity, or an absurdity, but whoever reads this release carefully will be able to understand how this idea is not only sensible, but also suitable to guarantee good results.
We gradually enter into the heart of the matter, starting to remember that the Founding Fathers of the theory of heat are essentially two:
The British physicist William Thomson, later Lord Kelvin (1824–1907) and the German physicist Rudolf Julius Emmanuel Clausius (1822–1888).
The theory of heat developed by them is divided into two parts:
1) the First Principle of Thermodynamics, about which there is no doubt, as it is based on a “crucial experiment” (or Experimentum Crucis) made in 1847 by the British scholar James Prescott Joule (1818-1889);
2) The Second Principle of Thermodynamics, characterized by the fact that it is not based on a crucial experiment, but only on the simplistic finding that in Nature heat always get out of hot bodies and moves towards less hot bodies.
Starting from this observation, the Founding Fathers put at the basis of the second part of the theory of heat (the Second Principle of Thermodynamics) a proposition which they considered indisputable (what is called an axiom), which was later shared by the Scientific Community.
Over time, scholars, scientists, authors of Thermodynamics textbooks, etc. have elaborated a number of different versions of this axiom.
Since these different versions of the axiom are considered equivalent by the Scientific Community, in the following we will often talk about “axiom” without specifying the author.
However, let’s remember which are the main versions of the axiom.
In 1851, KELVIN publishes a memoir entitled
“On the Dynamical Theory of Heat, with numerical results deduced from Mr. Joule’s equivalent of thermal unit, and M. Regnault’s observations on steam”, (Transactions of the Royal Society of Edinburg, March, 1851 and Phil. Mag. IV, 1852)
This memory is traceable to the following web address:
https://www3.nd.edu/~powers/ame.20231/kelvin1851.pdf
In this memoir KELVIN expresses his version of the axiom:
“It is impossible, by means of inanimate material agency, to derive mechanical effect from any portion of matter by cooling it below the temperature of the coldest of the surrounding objects.”.
The axiom adopted by CLAUSIUS is the following:
“Heat can never pass from a colder body to a warmer one without some other change connected with it at the same time.”.
This axiom is expressed in a memoir published in German in 1854, then published in English in 1856 with the title “On a modified Form of the second Fundamental Theorem in the Mechanical Theory of Heat” – (The London, Edinburg and Dublin Philosophical Magazine and Journal of Science [Fourth Series] August 1856).
This translation can be consulted, free of charge, at the following web address:
www.biodiversitylibrary.org/item/20044#page/95/mode/1up
It was KELVIN himself who affirmed (in his own memoir) the equivalence of his axiom with that of CLAUSIUS.
The axiom expressed in 1903 by the great German physicist Karl Ernst Ludwig Planck (1858-1947) is of particular interest:
“It is impossible to build a machine that, working in one cycle, it is able to produce no effect except the raising of a weight and the cooling of a heat-reservoir.”
This axiom is contained in the work of PLANCK “Treatise on Thermodynamics”, Longmans, Green, and Co, London, 1903.
In particular, here we consider the translated version in English of this work, Ed. Dover Publications, Inc., fifth edition of 1917, page 89, traceable to the following link:
https://archive.org/details/in.ernet.dli.2015.154233
The Scientific Community has also accepted the combined Kelvin-Planck axiom:
“It is impossible to build a machine that works in one cycle, it is able to produce another effect than that of the extraction of heat from a source and the production of an equivalent amount of work.”.
As you can guess, these are propositions that seem designed to not immediately perceive the brutal concept that they substantially express:
“It is impossible to construct a thermal machine capable of absorbing heat from a body, and converting it into mechanical work, although it lacks a heat sink (or a heat radiator).”.
This thermal machine would absorb heat and convert it into work without releasing heat to a heat sink, so it would therefore have a 100% efficiency.
In short, according to the axiom, Man could not build a thermal machine without a heat sink, or a thermal machine with a 100% yield.
To simplify the analysis, we agree that the axiom is expressed by the brutal proposition above.
If we note that this is not an axiom relating to Natural phenomena, but rather an apodictic affirmation that imposes a limit to the improvement that mankind can bring to the technology of thermal machines, we cannot help but recognize that, in establishing it, the Founding Fathers carried out a real reversal with respect to what the Scientific Method provides.
Usually, it is science that conditions what can be achieved technologically, but the Founding Fathers decided to do the opposite: the technology of the second half of the 19th century was to condition the theory of part of the heat theory for all future centuries.
Without fear of denial, it can be affirmed that this was a first error of formulation (therefore of Logic) committed by the Founding Fathers.
We need to take a small step back to understand the motivations that led to the formulation of the axiom, and to remember that before JOULE’s experimentum crucis the Scientific Community believed in the Caloric theory, according to which heat was an immaterial fluid, invisible and immutable in quantity, which produced work only when it flowed from a higher temperature to a lower one while crossing a thermal machine.
According to this theory, there was therefore a need to supply two temperatures to the thermal machine so that the Caloric could flow through it.
When JOULE’s experimentum crucis demonstrated the misconception of the Caloric theory, the Founding Fathers had to create the currently existing heat theory, and they did so by making the smallest possible change to the Caloric theory, and therefore continued to believe that it was necessary to dispose of two temperatures for a thermal machine to start working.
Only one change was introduced: the heat no longer remained unchanged in quantity (as the old Caloric theory predicted), but while it moved from hot bodies to less hot bodies, crossing the thermal machine, it decreased in quantity to compensate for the work produced.
The fundamental point to understand is that the Founding Fathers elaborated their axioms, which basically foresee the existence of two operating temperatures, without knowing the thermionic effect, since it was discovered after their death.
Therefore they could not conceive the idea that a thermal machine can exploit any ambient temperature as a higher temperature to spontaneously evaporate the electrons, while it is possible to do so (as we shall see) that the lower operating temperature is created by itself due to evaporation of electrons !
The next Figure 1 is a useful tool to understand what the limit imposed by the axiom to the technological improvements that can be made to of thermal engines consists of.

Fig. 1
Figure 1 represents the section of a hypothetical thermal motor Et, which continuously absorbs heat (Q) from a spherical body that surrounds it, constantly generating mechanical work outside this spherical body through a connecting rod and crank system. The temperature of the spherical body (the heat source) is supposed to be, for example, 1000 °C and constant over time.
As can be seen, there is no heat sink (or heat radiator), and therefore the Et machine, if it existed and operated continuously, would completely exploit the heat absorbed by the hot source at 1000 °C and convert it entirely into mechanical work: it would attain 100% efficiency.
This thermal machine has been given the derogatory name of “Perpetual motion engine of the second kind” – derogatory, as everyone knows that Perpetual Motion is impossible !
Using terms now considered to be not exactly correct, it can be said that this thermal machine would only use the “Temperature” of the hot source of heat, not the “Temperature Difference” between hot source and a heat sink (which in fact does not exist).
The latter, in fact, is the operation of all the common thermal machines that produce mechanical work, which function between two operating temperatures, in accordance with the Second Principle of Thermodynamics.
This Principle provides that a thermal machine can only work if it is built in such a way as to absorb heat from a “source” (of heat) and release a part of this heat to a “heat sink”, as schematically shown in the following Figure 2.

Fig. 2
In Figure 2, the thermal machine A works according to the Second Principle of Thermodynamics: it absorbs the quantity of heat +Qs from the hot source, releases some of this heat –Qa to the heat sink, and converts the difference into the mechanical work La.
There are two inevitable consequences of operation according to this Principle:
1) a thermal machine must always have a heat sink;
2) a Temperature Difference must always exist between the hot source and the heat sink before the engine starts to work, otherwise it cannot start to absorb heat, and therefore it cannot start to convert this absorbed heat into mechanical work; furthermore, this temperature difference must be artificially maintained even during operation.
The Scientific Community, based on the aforementioned Principle, does not believe that the difference in temperature between hot source and heat sink is “caused” by the work produced by the thermal machine; or what is the same: it is not believed that the difference in temperature between heat source and heat sink is the “effect” of the mechanical work produced by the machine.
The axiom seems to be based on the following intuition: if the temperature of the machine Et is exactly equal to that of the environment that surrounds it, then the lack of a Temperature Difference (albeit infinitesimal) cannot cause the entry of heat from the source towards Et, and no amount of heat can escape from Et and go towards the heat sink, as this component is even absent.
Therefore, according to the concept nowadays shared by the Scientific Community, this type of engine cannot exist, not being able to convert into mechanical work a quantity of heat that cannot enter or even escape from it.
Attention ! sometimes intuition deceives.
As we said, this axiom made sense in the second half of the 1800s, because at that time the technological implications of the physical phenomenon known as the “thermionic effect” had not yet been fully understood: the property of a free electron to be spontaneously expelled from a metal due to its temperature (not by any Temperature Difference).
If the Founding Fathers had this awareness, they would most likely not have adopted the aforementioned axiom.
Unfortunately, the applications of the property possessed by the free electron to be spontaneously expelled from the surface of a metal (the thermionic tubes called “diode” and “triode”) were built in the early 1900s (1904-1906).
Furthermore, the idea of yielding the spontaneous emission of electrons to obtain electricity was advanced more than a century ago, in 1915 (W. Schlichter, “Die spontane Elektronenemission glühender Metalle und das glühelektrische Element”, Ann. Phys., vol. 352, no. 13, pp. 573–640, 1915), when the theory of heat conceived by the Founding Fathers was accepted by the Scientific Community.
It is true that in recent years some scientists have created, published and patented devices based on the thermionic effect that contradict the axiom.
For example the Xu Yelin non-bias pneumatic vacuum diode in 1988; the solid-state non-bias diode by Xu Yelin, Jiang Ling and Xu Qlang between 2000 and 2004, with as many as 10 patent applications filed by the Academy of Science of Beijing; then the Grafene self-charging battery due to Zihan Xu (Hong Kong Polytechnic University – Department of Applied Physics and Materials Research Center – Nanjing – China); Guan Tai (like the previous one, but also the State Laboratory of Mechanics and Control of Mechanical Structures – Nanjing – China); Yungang Zhou and Fei Gao (Pacific Northwest Washington National Laboratory – U.S.A); and Kin Hung Wond (Hong Kong Polytechnic University – Department of Applied Physics and Materials Research Center – Nanjing – China in 2012.
These devices are able to generate electric current of sufficiently high intensity for technological applications of practical utility.
But the reluctance of scientists to dismiss a Physics Principle that was accepted after an incalculable number of diatribes is well known, and therefore it is not surprising that these striking results were not enough to convince the Scientific Community to accept the idea that the axiom should be rejected.
Chapter 3 – Assessment of the incorrectness of the second principle of thermodynamics
The fact that the entire theory concerning the Second Principle of Thermodynamics is erroneous is easily verifiable in two different ways.
The first way of doing this verification consists in finding, experimentally, that the axiom which is the foundation of the Second Principle of Thermodynamics has no general validity, since at least one system that contradicts it can be built and operated.
This system is based on the spontaneous emission of electrons by metals (the thermionic effect).
The experiment that can be carried out, exploiting this natural phenomenon, demonstrates the erroneousness of the axiom that in 1851-1854 was placed as the foundation of the theory of the aforementioned Principle.
The experiment is so easy to carry out, that it can be repeated in educational laboratories, such as those of some Technical Institutes for Electronics.
Those who are not satisfied with the outcome of the experiment, can use a different method to convince themselves of the wrongness of the theory concerning the Second Principle of Thermodynamics.
According to this other procedure, we start from the assumption that the axiom is true, then a critical analysis of the reasoning of the Founding Fathers allows us to verify (with elementary considerations of Logic easily understandable by anyone) that the reasoning that gave rise to the theory of the aforementioned Principle on the basis of the truth of the axiom is incorrect.
In the following paragraphs these two different ways of ascertaining the incorrectness of the Second Principle of Thermodynamics will be described.
Should the Scientific Community acquire this awareness, it should be induced to totally modify the theory relating to the Second Principle of Thermodynamics, starting from completely different axioms than those considered so far.
Chapter 4 – How the assessment of the erroneousness of the second principle of thermodynamics can be useful to contrast the global warming
The phenomenon of global warming is closely related to the energy problem, which, in turn, is closely linked to the axiom which is the foundation of the current version of the Second Principle of Thermodynamics, as well as the demonstration (unfortunately wrong) of a fundamental theorem of this theory: the Carnot theorem.
Incidentally, it should be noted that the importance (although negative) of this theorem, if valid, would be to impose a limit on the maximum value of efficiency obtainable from thermal engines producing mechanical work. A corollary of the Carnot theorem would allow us to quantify the extent to which the maximum efficiency theoretically obtainable from a thermal machine should be lower than 100% – efficiency which should always remain well below 100%, reaching between 30 and 50% even for ideal thermal machines.
Going back to the point, it seems that it is increasingly clear that the main cause of global warming consists in introducing carbon dioxide into the atmosphere, and that the combustion of fuels to obtain energy makes an important contribution to this input.
The so-called “Alternative Energies” could reduce this type of input, but it is evident that not all these alternative energies avoid the introduction of carbon dioxide into the atmosphere, since many of them require the combustion of some type of fuel.
There is another fundamental aspect of the question to consider: the minds of scientists, engineers, politicians and all the rest of society are conditioned by a fundamental idea, albeit incorrect: believing that to get energy it is necessary to consume something irreversibly; for example, the transformation of chemicals that react with each other; the consumption of a burned fuel; the nuclear transformation of one or more natural radio-active elements; the disruption of a large territory to obtain hydraulic energy, etc.
Even solar energy consumes something: the soil – forces the perennial occupation of large spaces to install solar panels, while wind energy has a considerable visual and sound impact.
In short, in one way or another, each of these ways of forms of energy production causes some “irreversible” consequences.
The idea of irreversibility is closely linked to the second part of the theory of heat developed by the Founding Fathers: the one in which all natural phenomena (no one excluded) are considered irreversible (a concept that was derived directly from the axiom, as we will soon see).
The idea of irreversibility is also linked (do not be frightened !) to the consequent Principle of the Increase of Entropy – of which we will shortly speak in a very simple and intuitive way.
Nowadays, it is easy to meet up with some learned people who, speaking in general about the state of the Earth, cite the Principle of the Increase of Entropy.
Citing this physical principle, the scholarly interlocutor intends to refer to the irreversibility of natural phenomena, which “increase the disorder of things” – concept that scientists link to the increase of an entity called “Entropy”.
But what would this entropy consist of ?
Entropy is a physical entity that was defined towards the second half of the 1800s by the Founding Fathers of the theory of heat, starting from the Carnot theorem.
If therefore it were true (as it is argued here) that the axiom has no general validity and that even the proof of Carnot’s theorem is affected by several Logic errors, it would follow that the Principle of the Increase of Entropy could also be incorrect, or not completely correct.
But the practical consequences on the fate of entropy that derive from the aforementioned arguments are of little importance to most people, who are instead very concerned about Global Warming.
Having said all this, we can begin to understand how and why ascertaining the erroneousness of the Second Principle of Thermodynamics may allow mankind to counteract global warming.
Taking note of the erroneousness of the axiom and the errors contained in Carnot’s theorem, it becomes impossible, for theoretical physicists, to exclude that mankind can build the engine prohibited by the axiom (the engine without heat sink), which would give access to the “True Alternative Energy Inexhaustible and non-polluting”- the “production” of which would not involve the emission of carbon dioxide into the atmosphere.
For greater clarity: if the axiom, the Carnot theorem and the Principle of the Increase of Entropy are not correct, then it is possible to design and build the thermal engine without a heat sink. This engine could work without needing two heat sources with different temperatures, needing just one body having a “Whatever Temperature”, which could be the temperature of the terrestrial environment in which the machine would be located.
This engine would make it possible to “exploit” that energy present in every place of the Earth and the Universe, which consists of the sum of the inexhaustible vibratory energies possessed by each atom or molecule of the material world (for more information, set up an Internet search by typing “Brownian Motions”).
If the Scientific Community took note of the aforementioned errors, the idea that energy can be “produced” (a concept that implies the appearance of some type of sub-product that must be disposed of in the environment) would in turn be mistaken; instead, the idea that it is possible to “exploit” or “intercept” the energy that is in constant circulation, without such exploitation implying the introduction of any type of gas into the atmosphere, would make sense.
Ultimately, if the Scientific Community took note of the wrongness of the Second Principle of Thermodynamics, the heads of the microelectronics industries could authorize the planning of studies and experiments concerning electric current generators composed of a series of Silicon “wafers”, with surfaces treated according to the procedure invented by the Chinese physicist Xu Yelin and described in the patent applications filed by the Institute of Biophysics, Chinese Academy of Sciences, Beijing.
If these studies were successful, Humankind would have the opportunity to tackle the fight against global warming much more efficiently than the palliatives currently implemented by Nations, since that type of electric current generator would extract the corresponding energy from the vibratory motions that agitate the set of atoms and molecules that constitute the terrestrial environment – what we could call the “environmental heat” (a concept considered non-scientific today).
Nowadays, however, studies and experiments of this kind cannot be undertaken by the microelectronics industries: anyone who manifested the initiative to propose them, would at least risk being ridiculed if not actually fired. In fact, his initiative would be rejected without appeal by any scientist.
Chapter 5 – Experiment that shows the wrongness of the axiom of the second principle of thermodynamics
In this Section we describe the rational of an experiment based on the thermionic effect, which shows that the axiom which is the foundation of the theory concerning the Second Principle of Thermodynamics has no general validity.
The basic idea of this experiment is due to several scientists, researchers and scholars, who have carried out experiments and deposited patents related to systems based on the thermionic effect – ideas that have been made public in recent years (1988, 2000, 2004 and 2012).
In the following we will explain how to repeat a simplified version of the experiment created by the author and described in his eBook “Unfinished Book on the Energy of the Environment” – click on the following link to read the first 10% of the book for free.
Before illustrating the operation of this experiment based on the thermionic effect, we must first of all remember how thermionic valves work (devices used since the early 1900s for the construction of radio or receiver devices, radar, television, etc.).
These thermionic valves consist of a glass tube (this is why they are also called “Thermionic Tubes”) hermetically sealed and emptied of air, and by various internal electrodes. See Figure 3.

Fig. 3 – 3Q4 thermionic tube and relative electric scheme
In normal use, the electrodes of a thermionic tube are powered by electric current so that they can perform the intended functions.
One of these electrodes (the filament) is made of metallic materials treated in such a way as to give it the property of emitting a consistent flow of electrons from the surface.
Another electrode (the plate) is made of metallic materials that are unable to emit consistent flows of electrons from the surface. This electrode serves to attract and capture the electrons emitted by the filament.
Another electrode that is unable to emit consistent flows of electrons is the control grid; it is interposed between filament and plate and, in normal operation, serves to control (or vary) the flow of electrons captured by the plate.
Some electronic tubes (like the one in Figure 3) have two or even three intermediate grids between filament and plate, to perform functions that are not of interest here.
In order for a thermionic tube to perform the functions for which it was conceived (normal operation), its filament must be made incandescent, and this is achieved by running the filament by an electric current.
The higher the filament temperature, the higher the flow of electrons that comes out of its surface.
However, there is no lower limit of temperature below which the emission of electrons stops completely; the lower the temperature, the lower the electronic emission from the metal surface, so a metal emits a flow (even if small) of electrons even at an ambient temperature of 20 ° C, and the experiment described here is based precisely on this property.
Therefore, even if the filament is not supplied with an electric current (for which the filament remains cold), and if grid and plate are not connected to an electrical power supply, the filament still emits a certain flow of electrons inside the empty tube.
That said, here it is proposed to operate these thermionic tubes in a completely abnormal way: without feeding them in any way with electric current, and indeed making them become electric current generators. The temperature of the filaments is increased by heating the entire tubes.
Therefore, even in these conditions the free electrons are “fired” away spontaneously from the filament – but where does the energy needed to produce this expulsion come from?
As mentioned above, the energy needed to expel a free electron from the surface of a metal comes from microscopic vibratory motions that continually agitate, in an absolutely chaotic way (see “Brownian motions”), the atoms and molecules that make up the metal.
Consider a single free electron that lies just below the surface of the metallic filament of a thermionic tube; this electron continually undergoes random collisions by the metallic atoms that surround it, since they vibrate chaotically in every direction. The characteristics of these random collisions are determined only by the average temperature of the metal.
Sometimes it happens that the vibrations of the atoms add up in the impact against a particular free electron, giving it enough energy to allow it to jump out of the metal surface.
When this happens, the filament loses a very small amount of energy, and therefore its atoms shake with less intensity so that the filament cools down, albeit very little.
These concepts are not the result of a theory developed by the author, but are the physical reality of electronic emission resulting from experiments conducted and recognized by the Scientific Community.
The electrons come out in all directions starting from the filament, and since some of them can be directed towards the grid and / or the plate, then a certain fraction of these electrons can fall on these electrodes, even if they are not connected to an electric generator.
Grid and plate are thus loaded with electrons, which are notoriously negatively charged, so that these electrodes take negative electrical charges; the filament, on the other hand, becomes positive.
But the negative charge of each of these two electrodes goes against the further capture of electrons, given that electric charges of equal sign repel each other, therefore in grid and plate an electrical balance is created which is “static”; to this equilibrium corresponds a certain difference in electrical potential (voltage) between grid and filament, and another value of tension between plate and filament.
The equilibrium is of a static type, since the motions of all the electrons remain blocked: the electrons captured (and blocked) on grid and plate, prevent (block), with their negative electric charge, the entry of other electrons fired away from the filament.
We note that in these conditions of static electric equilibrium, the whole system is at the same temperature, absolutely identical for all the components of the thermionic tube: the temperature of the surrounding environment.
(For those unfamiliar with electricity, it should be noted that a “resistor” is, for example, that component of an electric heater that heats up when it is crossed by electric current)
If in these conditions of static equilibrium a resistor is connected between plate and filament, and another resistor between grid and filament, as in the following Figure 4, the static equilibrium is destroyed, for which the voltages that were previously created on grid and plate begin to run electrons (generate electric current) through the respective resistors.

Fig. 4
The fact that a part of the electrons captured by the grid or the plate flow away from them and cross the resistors, decreases the electrical voltage with which each of these two electrodes was charged. Since this lower voltage value causes a smaller electric repulsion field for the electrons coming from the filament, then other electrons emitted by the filament can again penetrate inside grid and plate.
Thus, a double flow of electrons is initially created: the first through the resistors and the second in the vacuum, between the filament and the two electrodes (grid and plate). At first, we have said, but what conditions must be verified so that these electron flows remain constant over time ?
To answer this question, we recall that the energy needed to expel an electron from the filament is provided by the microscopic chaotic motions of atoms and molecules of the filament, while the average energy of atoms and molecules of the filament is proportional to its temperature, so if the average energy of the filament decreases, because it emits electrons, then the average filament temperature must also decrease, and consequently the flow of electrons it emits must also decrease.
Therefore, so that the filament temperature does not decrease too much, it is necessary that it receives heat. Then the question we asked ourselves before turns into another: where can this heat come from, so that the flows of electrons remain constant over time ?
To answer this other question, we observe that if some electrons impact with a certain speed on the grid and on the plate, then these impacts tend to heat up the grid and the plate; but how much ?
If the whole of grid and plate heating were able to exactly compensate for the filament cooling, then the internal temperature of the thermionic tube would remain constant on average and equal to the initial ambient temperature. This would imply the arrest (sooner or later) of electron flows, because otherwise the principle of energy conservation would be violated.
In fact, if electricity continues to flow through the resistors, the thermal energy that they would disperse in the environment would come from nothing.
But there is the hope that the cooling that undergoes the filament is greater (in absolute value) than the whole of grid and plate heating.
In fact, as we have said, grid and plate are charged with electrons, and the electric field generated by them slows down the motion of the electrons that are directed towards these two electrodes arriving from the filament.
Therefore, the kinetic energy of the electrons that are successful in penetrating grid and plate, is inferior (they are less fast) to that which they possessed when they abandoned the filament, so their impact on the grid and plate must cause a slight heating.
This means that when the resistors are connected, the filament temperature must decrease to a greater extent than the heating of grid and plate – overall, cooling must prevail inside the thermionic tube.
The removal of heat from the filament, must determine inside the tube a lowering of the average temperature compared to that of the environment, and this can allow heat to flow spontaneously from the environment to the inside of the thermionic tube, with the result that electric currents can continue to flow through the resistors.
But the temperature inside the tube cannot decrease without limits. In fact, the progressive increase in the difference in temperature between the exterior and interior of the tube increases the flow of heat coming from the environment which is directed towards the inside of the tube itself, whereby at a certain point the internal temperature of the tube must be stabilized at a value lower than that of the ambient temperature.
In reality there are many temperatures that must be created in this system: one on the filament, one on the grid and finally the last on the plate.
These temperatures, different from each other and different from the ambient temperature, cannot be the “cause” of the electric currents flowing in the resistances; but they must instead be the “effect” of these currents.
These differences in temperature do not exist “before” the system begins to produce electricity, but they are formed “after” the system has started to work by itself.
This is the Copernican revolution that can spring from the success of the experiment !
The mistake that has been maintained to this day is to believe that heat can never be absorbed by a thermal engine, and converted into mechanical work by it, if we initially do not connect the engine to two material bodies with different temperatures before it starts working, and we do not maintain this difference in temperature even during the entire period of operation.
The system described above, instead, if it worked according to the description, would become a thermal engine that makes electric current flow inside the resistors, working, however, in exactly the opposite way to all the engines that work according to the Second Principle of Thermodynamics: it is true that the engine would need a “Heat Source”, which would be constituted by the surrounding environment, but the engine would not work thanks to a Temperature Difference that exists before the engine itself starts to work.
This particular thermal machine would have no heat sink, which, instead, for machines that work according to the Second Principle of Thermodynamics, must be present both before the machine starts to work, and during its operation, to ensure the existence of the lowest temperature.
On the contrary, the particular thermal engine mentioned above would be able to generate the lowest temperature on its own, which would be created after the system started generating electricity.
What we imagined would be a revolutionary thermal engine.
We would have a system that runs electric current through the resistors (which would then heat up), even though it is not powered in any part by an electric generator, and in which the electric current that would flow through the resistors would be generated only thanks to the “Temperature” of the filament, not thanks to any pre-existing “Temperature Difference”.
The energy that would be dispersed in the environment by the resistors would not come from nothing, and the Principle of Conservation of Energy would not be violated.
Ultimately, the thermal energy would “circulate” continuously according to the following path: Environment → Filament → Resistors → Environment (again) and so on.
To conceive a really feasible experiment based on these premises, the construction must be simplified by eliminating, for example, the resistor connected to the plate.
Moreover, to increase the output voltage, a certain number of tubes of the same type can be connected in series, as shown graphically in the following Figure 5.

Fig. 5 Fig. 1
If we now compare Figure 1 with Figure 5, we note that the two engines have the same configuration.
Both have no heat sink and the only difference is that the first would produce mechanical energy while the other one would generate electricity by circulating the electric current I through the load resistor R.
The engine of Figure 1 is the one forbidden by the axiom, while in Figure 5 the one that could actually exist is represented when the described experiment worked according to the methods indicated above.
Chapter 6 – Results of the experiment described in the eBook
Before setting out the procedure to follow to perform a simple experiment that demonstrates the non-generality of the axiom, it is advisable to give an account of the results obtained with the experiment described in the above-mentioned eBook.
Although this experiment presented some anomalies with respect to what was expected to be found, its outcome was positive: the voltage that developed at the ends of the load resistor connected in parallel to the thermionic tubes and to the electronic voltmeter, has always remained different from zero and has reached small but significant values.
Furthermore, this voltage varied considerably as the oven temperature varied.
The following Figure 6 shows two typical Voltage/Temperature diagrams obtained during the various repetitions of the experiment. Notice in the graph on the right the cooling curve, highlighted with the triangular points.

Fig. 6
The anomalies found are the following:
1) For relatively low temperatures (less than 200≈300 ° C), the voltage developed at the ends of the load resistor, instead of assuming a negative value (as expected), sometimes showed positive values. The expected negative values took over steadily at higher temperatures;
2) For relatively low temperatures, there was an oscillation of the measured voltage values while the temperature increased;
3) The repetition in the following days of the experiment did not produce the same voltage values, at the same temperature, even if nothing had been changed in the system.
The existence of this unexpected behaviour does not imply that the experiment is not repeatable and significant.
There are few criteria to be taken into consideration to establish whether the experiment is repeatable and meaningful.
A) First of all, one must be sure that the voltage developed at the ends of the load resistor is determined only by the thermionic tubes and not by other foreign elements. To be sure, all the electrical connections between the system and the electronic voltmeter have been shielded and grounded. To prevent the thermoelectric effect (or Seebeck effect) from generating voltages, conductors made of the same metal (Silver) were used both for the internal connections between one pentode and the other, and for the connection of the external electronic voltmeter to the series of thermionic tubes. On the other hand, the thermoelectric effect cannot at first generate positive tensions and then negative tensions;
B) Then you need to be sure that the voltage value is a function of the temperature of the oven. The measures have shown that this happened. The fact that the voltage changed strongly with temperature, gave a further guarantee that the voltages measured did not depend on electrical interference;
C) Furthermore, it must be ensured that the voltages measured do not depend on the presence of the natural radioactive elements which sometimes are introduced in the filament to favour the electronic emission. Since radioactivity is not influenced by temperatures of the order of 500 ° C, the fact that the value of the measured voltage depended on the temperature, showed that the tension could not have been generated by natural radioactive elements incorporated in the filament;
D) Finally (decisive criterion) it must be verified that the voltage developed at the ends of the load resistor is different from zero (either negative or positive). The circumstance that the voltage developed at the ends of the load resistor assumes minimum values cannot imply that the experiment does not demonstrate the wrongness of the axiom. If the axiom had general validity, the voltage present at the ends of the load resistor should always remain exactly equal to zero at any temperature. On the contrary, the experiment showed that such voltage was never exactly equal to zero, even if it has taken minimum values, such as thousandths, hundredths, or even tenths of Volt for temperatures close to 500 ° C.
“For a theoretical spirit a millionth is like a million, it means that it is not zero, where does it come from ?”, so the physicist Roberto Germano expressed this concept on page 151 of his book “AQUA – L’acqua elettromagnetica e le sue mirabolanti avventure” (AQUA – Electromagnetic water and its amazing adventures), Ed Bibliopolis, 2006.
It is true that in the passage from positive to negative values the voltage actually existing at the ends of the load resistor must necessarily have assumed the value zero, but we have seen from experience that this passage has always been very fast with varying temperature, and the zero value, predicted by the theory of the Founding Fathers, was the most unstable of all the values measured in correspondence of sufficiently high temperatures.
By pure tutiorism, we could put forward some hypotheses to explain the unexpected behaviour mentioned above – for example, that they could be the result of the impact of the electrons emitted by the filament with gas residues present inside the thermionic tubes, which, in fact, are never completely emptied of air.
But we must keep in mind that the concept of “anomaly” should have little meaning in Science, when dealing with an experiment. Nature does not always behave as the theories currently in force would expect it to happen.
For example, if a hypothetical experiment carried out by various independent experimenters demonstrated, without a shadow of a doubt, that an event that occurs on Earth determines another event on the Moon with a time interval equal to zero, it would not be lawful to reject the validity of the experiment a priori, justifying the denial with the violation of Einstein’s theory of relativity, according to which this time interval should be greater than one second.
In short, the last word can never be that of theory, but the one that springs from the work conducted by independent (of everything and everyone !) experimenters.
The following link allows you to view the video of the experiment (New Esperiment…). On each page, it is convenient to click on the “PAUSE” button to have time to read the text it contains.
https://www.youtube.com/channel/UCJj0yC24yVCBqpWJJ1_RhIw

Chapter 7 – The wrongness of the theory related to the second principle of thermodynamics – The logical errors
Introduction
As mentioned above, not only can an experiment be performed that shows that the axiom which is the foundation of the theory concerning the Second Principle of Thermodynamics has no universal value, but it can be shown that there are numerous errors of Logic in the reasoning that generated this theory starting from the axiom.
The complete demonstration of these errors can be found in the eBook mentioned above; it is the result of a critical analysis of the main memoirs published in the second half of the 19th century by the two Founding Fathers of the dynamic theory of heat: William Thomson, later Lord Kelvin (1824–1907) and Rudolf Julius Emanuel Clausius (1822–1888).
The eBook highlights the fact that the same errors are also found in modern physics textbooks, and therefore modern generations of students are forced to learn partially incorrect notions about the second part of the theory of heat.
The critical analysis contained in the eBook was designed to be understandable by anyone, even by those who do not have a good knowledge of mathematics and physics, given that the errors highlighted are of Logic.
The following is a simplified summary of this critical analysis, which allows readers to realize the actual existence of the aforementioned errors without having to consult the eBook.
To enter the topic of errors present in the second part of the classical theory of heat, remember again that in the second half of the 1800s the composition of the atoms was not known, and therefore the technological consequences of the property of free electrons of being expelled from the surface of metals due to the temperature alone could not be imagined.
Therefore, no scientist of that time had the opportunity to realize that the Founding Fathers had introduced an axiom that could be contradicted by an experiment.
As regards most of the Logic errors that the author attributes to the Founding Fathers, they were detectable by scholars of the second half of the 1800s, and it is incomprehensible that even modern scientists continue not to notice them, with the sole exception of those unheard Rationalists who are dedicating themselves to the construction of a Rational Thermodynamics.
Chapter 7.1 The scheme of Carnot’s theorem
Before describing the aforementioned errors of Logic, it is appropriate to recall how the Founding Fathers devised the proof of Carnot’s theorem – theorem that led the theory of the Second Principle of Thermodynamics to a condition of complete confusion and irrationality.
This paragraph contains a summary of the reasoning that is generally used to demonstrate Carnot’s theorem, while in the next paragraphs the errors that make the demonstration of this theorem not shared will be highlighted.
To simplify this summary, we will consider the proof of Carnot’s theorem according to the scheme devised by KELVIN – the one described in his memoir of 1851.
Keep in mind that even the proof of Carnot’s theorem according to the scheme devised by CLAUSIUS (reported in his memoir of 1854) is affected by the same errors, as explained in the aforementioned eBook.
The demonstration of the Carnot theorem according to KELVIN’s scheme, is achieved through a proof by reductio ad absurdum, where the absurdity would consist in the violation of the axiom conceived by KELVIN himself:
“It is impossible, by means of inanimate agents, to obtain a mechanical effect from any portion of matter by cooling it below the temperature of the coldest of the surrounding objects”.
In simpler terms, the absurdity would consist in the existence of a thermal engine that absorbs heat and produces work even if it lacks the heat sink (the coldest of the surrounding objects) – engine also known as “Perpetual Motion of the Second Kind”.
The reasoning connected to the proof of the Carnot theorem, according to KELVIN’s scheme, foresees imagining the existence of two heat sources, one hot (the furnace) and the other cold (constituted by the heat sink); moreover, it is foreseen the presence of a first ideal thermal machine that works between these two temperatures, where every transformation that takes place in this machine is reversible, so such machine (Rev) is overall reversible.
Then we imagine the existence of another non-ideal or “whatever” thermal machine, always working between the same temperatures as before, where however some transformations that take place in this other machine are irreversible, for which this machine (Irr) is overall irreversible.
Since the first machine (Rev) is reversible, it can be operated inverted (as a refrigerator), so it absorbs some mechanical work that is consumed to move heat from the cold body (the heat sink) to the hot one (the furnace); the irreversible machine (Irr), on the other hand, is operated normally, like a motor, so it takes heat from the hot body, returns a part of it to the heat sink and transforms the difference into mechanical work.
It is later imagined that these two machines are connected to each other in order to work in opposition: the work produced by the irreversible machine is used to make the reversible machine work like a refrigerator.
At this point, the reasoning is to formulate the following hypothesis:
“There is an irreversible machine that is more efficient than the reversible machine.”.
Figure 7 below shows the system of the two opposing machines sized to comply with the hypothesis.
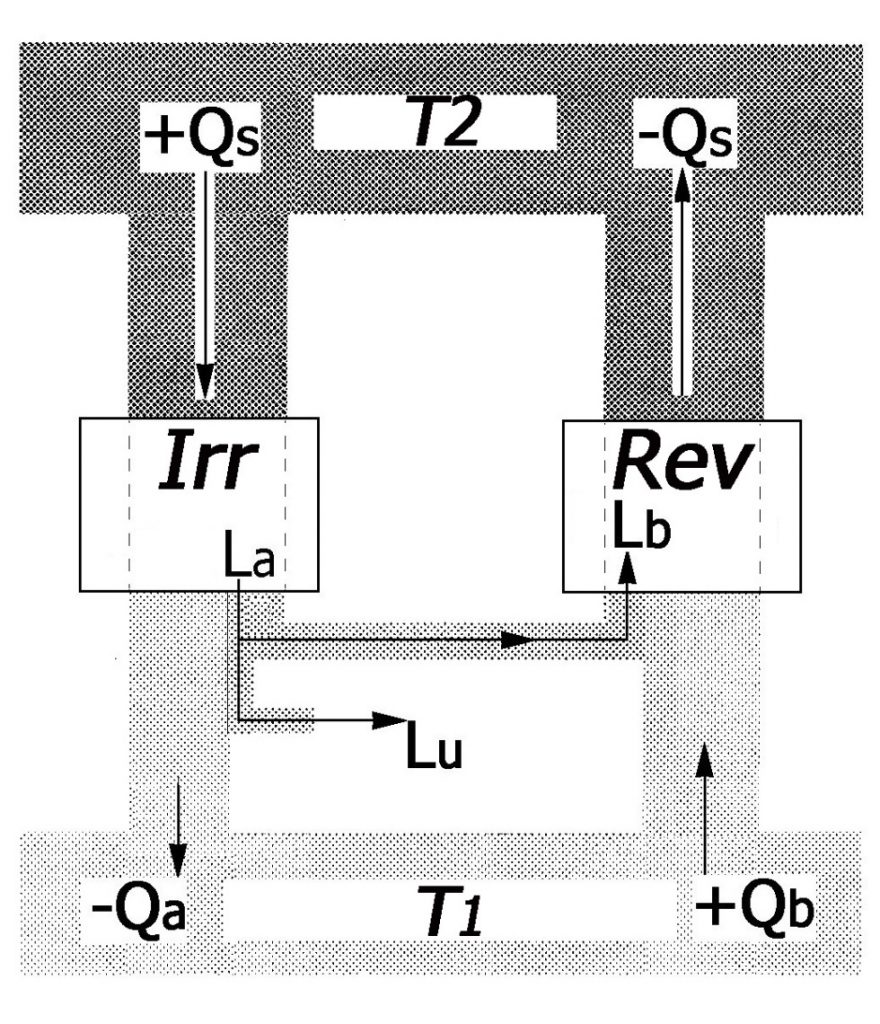
Fig. 7
As shown in Figure 7, if the irreversible machine (Irr) had a higher efficiency than the reversible one (Rev), the combination of these two machines would be able to produce the useful mechanical work (Lu) at the expense of the heat taken away (attention! not given to) from the heat sink (the coldest of the surrounding objects), which therefore would cool down even more.
In fact, as can be seen in the Figure, the amount of heat +Qb that the Rev machine would extract from the cold source T1 (the heat sink), would be greater (in absolute value) than the quantity of heat –Qa, that the Irr machine would release to the same heat sink; overall, the heat sink would be subject to continuous heat removal and would cool down more and more.
This constant cooling of the heat sink would constitute (according to the theorists) an absurdity, as it would represent precisely what is forbidden by the axiom.
Therefore, this kind of reasoning leads theorists to conclude that this complex machine cannot exist, since it would allow us to violate the holy axiom.
But since the existence of such a complex machine would be allowed only by the existence of an irreversible machine more efficient than a reversible one, then the reasoning is concluded by saying that the irreversible machine cannot be more efficient than the reversible machine.
Attention ! Having reached this conclusion, the demonstration by reductio ad absurdum is terminated.
But this conclusion is not enough for theorists !
In fact, if the reasoning illustrated so far was acceptable (but we will see that it is not for some reasons), it would have been shown only that an irreversible machine cannot be more efficient than a reversible one (between the same operating temperatures), but this could not exclude that the irreversible machine could be equally efficient as the reversible one.
However, as already mentioned, the theorists cannot accept this eventuality, that would force them to give up the fundamental concept they share on the Second Principle of Thermodynamics: the presence of irreversibility in a thermal engine always decreases its efficiency.
To reach anyway the coveted conclusion, theorists must pretend that the demonstration by reductio ad absurdum has not already ended, so they add that the equality of the efficiency is valid only when the irreversible engine becomes reversible.
This reasoning cannot be shared for the following reasons.
Chapter 7.2 – The second logical setting error
We have previously said that the Founding Fathers made a first error of Logic when they decided that the technology of the second half of the 1800s should influence the second part of the theory of heat for all future centuries.
A second error of Logic committed by the Founding Fathers was to derive from the axiom the concept that certain natural phenomena (or certain transformations) are irreversible.
This mistake in setting the theory concerning the Second Principle of Thermodynamics was introduced by KELVIN into a memoir published in 1852, entitled “On a Universal Tendency in Nature to the Dissipation of Mechanical Energy”, where he modified and perfected his idea about the concept of “irreversibility”.
This publication can be read for free at the following link:
https://www3.nd.edu/~powers/ame.20231/kelvin1852.pdf
In this memoir, KELVIN provides a clear indication of what would be irreversible or dissipative transformations, as a necessary consequence of the axiom. KELVIN writes, in fact (p. 511-512):
“The following propositions are laid down regarding the dissipation of mechanical energy from a given store, and the restoration of it to its primitive condition. They are necessary consequences [notice – Ed.] of the axiom, "It is impossible. by means of inanimate material agency, to derive mechanical effect from any portion of matter by cooling it below the temperature of the coldest of the surrounding objects." I. When heat is created by a reversible process (so that the mechanical energy thus spent may be restored to its primitive condition), there is also a transference from a cold body to a hot body of a quantity of heat bearing to the quantity created a definite proportion depending on the temperatures of the two bodies. II. When heat is created by any unreversible process (such as friction), there is a dissipation of mechanical energy, and a full restoration of it to its primitive condition is impossible. III. When heat is diffused by conduction, there is a dissipation of mechanical energy, and perfect restoration is impossible.”
Even in modern Thermodynamics textbooks this setting error can be found, and this provides a first proof of how the errors of the Founding Fathers are still shared by most of the Scientific Community.
It is easy to explain why deriving the concept of irreversibility of certain natural transformations from the axiom is a Logic error: the axiom is the starting point for founding a new theory (replacing the obsolete theory of Caloric) concerning a certain behaviour of heat, but we must consider the fact that the axiom chosen does not concern the behaviour of heat in Nature, but imposes a limit to the technologies that mankind can develop.
If, therefore, certain natural transformations are defined as irreversible as necessary consequence of such an axiom, two non-homogeneous conceptual entities are mixed: irreversible transformations for what Nature can never do, and transformations that mankind cannot reverse using the technologies allowed by the scientific knowledge of the moment.
Even today accepting this mixture of non-homogeneous concepts continues to maintain confusion in the theory based on the axiom, since the technological abilities of mankind related to the axiom remained those of the second half of the 1800s, while in the following 160 years the progress of science has allowed the achievement of highly refined technologies.
This distinction was never expressed in the theory relating to the Second Principle of Thermodynamics, since all the transformations that actually occur (both natural and those caused by man) have been considered irreversible, including heat conduction.
The following consideration makes us understand how this distinction should instead be introduced: there is no doubt that Nature is not able to reverse the conduction of heat, based on the definition of irreversibility shared today, but if there was a Perpetual Motion engine of the second kind, then a Physicist or an Engineer could use this type of engine to reverse this transformation without producing any change in the rest of the universe.
As we shall see in the next chapter, this setting error determines one of the reasons why the thesis that theorists would like to demonstrate for Carnot’s theorem is not actually obtainable.
Chapter 7.3 – First reason of unacceptability of the demonstration of the second theorem: The multiple use of the axiom
Referring to the contents of the previous Paragraph, we observe that the axiom is used several times in the proof of Carnot’s theorem: a first time (implicitly) to establish that the transformations making up the first machine are reversible; a second time (implicitly) to define that the transformations making up the second machine are irreversible; a third time (explicitly) to declare that the described complex machine cannot exist because it would allow us to violate the axiom.
However, it is not permissible, in a given reasoning, to invoke the same axiom several times to derive subsequent deductions. The multiple application of an axiom in the same reasoning allows the tautological demonstration of any proposition.
Chapter 7.4 – Second reason of unacceptability of the demonstration of the second theorem: The impossibility of using the proof by reductio ad absurdum
We begin to observe that in the proof of the Carnot theorem we find a use that is not allowed of the proof by reductio ad absurdum.
This kind of proof is based on the (Aristotelian) Principle of the Excluded Middle (in Latin: Tertium non datur).
The use of this principle was not contested by the Mathematicians of the second half of the 1800s, when the Founding Fathers conceived the theory of heat. This dispute was advanced later, in the early 1900s, by Constructive Mathematicians, such as the Intuitionist Mathematicians, of which Luitzen Egbertus Jan Brouwer (1881-1966) was the forefather, when by then the Scientific Community had accepted the heat theory created by the Founding Fathers.
See, for example, the work by Arend Heyting “Intuitionism; an Introduction”, North Holland Publishing Company – Amsterdam-London, 1966.
The Principle of the Excluded Middle can be applied only when in the system under consideration there are two (and only two) cases in mutual opposition. If it is recognized that one of the two cases is absurd or impossible, the only possibility that remains (tertium non datur !) is that the opposite case is true.
In reality, there are three possible cases in the physical system relating to Carnot’s theorem. In fact, the efficiency of a thermal engine may be greater, equal or lower than another.
Conclusively, in presence of three possible cases, the principle of the excluded third cannot be used to draw from the Carnot theorem the conclusion desired by the theorists, since it is not logically possible to conceive a single opposite of two different cases.
Chapter 7.5 – Third reason of unacceptability of the demonstration of the second theorem: The reductive hypothesis
As we have previously noted, the theorists have adopted the following reductive hypothesis to continue to use the principle of the excluded middle in the proof of the Carnot theorem: There is one irreversible thermal engine that is more efficient than another reversible that works between the same temperatures.
The theorists have adopted this reductive hypothesis to force the proof of the Carnot theorem towards the conclusion they wanted.
However, by thinking correctly, one can understand that this reductive hypothesis cannot actually allow the conclusion desired by the theorists, i.e., the one that would be fully consistent with the fundamental concept implied by the currently shared version of the Second Principle of Thermodynamics: the presence of irreversibility in the transformations that take place in a thermal engine decreases its efficiency, compared to the case in which the transformations are reversible.
The proposition that the theorists would like to be able to affirm, to remain adherent to the aforementioned coveted concept, is the following:
“No thermal engine can be more efficient, or even equally efficient than a reversible engine.”
As mentioned above, the reductive hypothesis has allowed theorists to use the principle of the excluded third in the proof of Carnot’s theorem, and to eliminate (momentarily) the third case: the one in which the irreversible thermal engine attains the same efficiency as a reversible thermal engine that works between the same temperatures.
But in reality this third case cannot be eliminated forever, if one wants to prove the thesis desired by the theorists, and then, in order to be able to affirm that this coveted thesis is true, it is argued, belatedly, that equality of performance occurs only when the irreversible thermal engine becomes reversible.
This argument, besides being late, is tautological and therefore unsustainable. In fact, once in that proof by reductio ad absurdum the hypothesis has produced the contradiction, the demonstration has ended, and the consideration that equality of performance is valid only when the irreversible thermal engine becomes reversible is a tautology that cannot transform the conclusion that can apparently be drawn form that reasoning:
“The irreversible engine cannot be more efficient than the reversible engine.”
in the conclusion that theorists would like:
“No thermal engine can be more efficient, or equally efficient than a reversible engine.”
Chapter 7.6 – Fourth reason of unacceptability of the demonstration of the second theorem: The reasoning is incomplete
Let’s go back to Figure 7, which is reported here for convenience.

(Fig. 7)
Looking at the Figure, we recall that theorists believe that the two opposing thermal machines can start to work only if two heat sources exist, i.e. two ideal bodies capable of maintaining constant the respective temperatures even when subjected to heat exchange: the source at temperature T2 and the heat sink at temperature T1.
Let us remember, above all, that theorists believe that the condition represented by the Figure is physically impossible, because the system of the two opposing machines would produce the useful mechanical work (Lu), absorbing the equivalent amount of heat (-Qa + Qb) from the heat sink (the coldest of the surrounding objects), violating the axiom.
Now it may be objected that the theorists who use KELVIN’s scheme prematurely interrupted the proof of Carnot’s theorem, since it is possible to introduce a “thermal resistance” between hot source and heat sink – with this change, the absurdity consisting in the violation of KELVIN’s axiom disappears, as is evident by observing the following Figure 8.

Fig. 8
Figure 8 shows a thermal resistance introduced between hot source and heat sink; the thermal resistance is calibrated and maintained on site so as to remove the exact amount of heat +QL = –Qa + Qb from the hot source, and transfer it to the heat sink, where this quantity of heat changes the algebraic sign (being heat transferred) and becomes –QL = –Qb + Qa. This amount of heat cancels the heat sink balance.
The introduction of thermal resistance does not prevent the two opposing machines from continuing to function as before.
But now it is no longer the heat sink that has to supply the heat necessary for the production of the useful mechanical work Lu (an event that constituted the absurdity), and therefore KELVIN’s axiom is no longer violated.
With this variant, the proof by reductio ad absurdum of the Carnot theorem with KELVIN’s scheme and axiom can no longer be completed (absurdity is missing).
Theorists could oppose a first argument to argue that a thermal resistance cannot be introduced: the subsequent introduction of thermal resistance would be equivalent to “changing the playing cards during the game”.
But this argument is unthinkable, since the heat that passes though the thermal resistance does not alter the transformations that occur in the two machines.
Theorists could oppose a second argument: the thermal resistance introduces an irreversible phenomenon (heat conduction). But even this objection is ineffective for two reasons:
1) If we consider the case in which the thermal resistance is introduced “after” having finished the proof by reductio ad absurdum, theorists cannot oppose this argument since they have established that heat conduction is irreversible as an immediate consequence of the axiom, so they would invoke the axiom for the second time, since they have already used it a first time to finish the demonstration.
2) If we consider the case in which the thermal resistance is included in the system at the beginning, then the axiom can never be invoked, because the heat balance of the heat sink remains zero permanently.
Ultimately, by “completing” the proof left incomplete by the theorists, Carnot’s theorem is no longer provable.
Chapter 7.7 – Conclusions on the classical version of Carnot’s theorem
Conclusively, the theorists “believe” to have demonstrated the thesis of the Carnot theorem that they desire, but theirs is only an illusion.
If instead the constructive Mathematicians were to deal with the Carnot theorem, I am willing to bet that they would agree that the various errors of Logic contained in the reasoning make the demonstration of theorists unacceptable, and that they have not shown anything about the maximum possible theoretical performance of a thermal engine that works between two temperatures.
Chapter 8 – The reversibility of heat conduction for Mankind
As we have seen, the axiom has been given a double meaning: not only has it been considered that it forbids the existence of a Perpetual Motion machine of the second kind, but it was also considered that heat conduction is an irreversible natural phenomenon as an immediate consequence of the axiom itself.
The conduction of heat is certainly always irreversible for Nature, but this is not necessarily true for man.
In fact, if there were a heat engine that works without a heat sink (a Perpetual Motion machine of the second kind), an Engineer or a Physicist could use it to reverse the natural phenomenon of heat conduction, while respecting the definition of reversibility currently shared.
The following Figure 9 illustrates the sequence of operations that could be performed with a Perpetual Motion machine of the second kind, to reverse the phenomenon of heat conduction.

Fig. 9
In Figure 9 (SEC I), the body C is initially in contact with the hot source, then the body is suddenly moved into the heat sink (SEC II) and therefore the phenomenon of heat conduction occurs.
Afterwards a Perpetual motion machine of the second kind heats up again the body C through a converter that transforms work into heat (SEC III), which works by viscous friction (VFC). This type of friction completely transforms work into heat.
When body C has again reached the temperature of the hot source, it can again be brought in contact with it (SEC IV), and the inversion is accomplished without altering anything in the rest of the universe.
Only man could realize the inversion of the phenomenon of heat conduction in this way; Nature, in fact, is not able to create the exact contrast of two opposing thermal machines, such as to create, on the whole, a Perpetual Motion machine of the second kind.
Chapter 9 – The modern version of the second principle of thermodynamics
Some of the most qualified authors of Thermodynamic textbooks follow a much more elaborate method, compared to the classic one of the Founding Fathers, in order to explain to the students the principle of the increase of entropy.
Probably, these authors use this sophisticated system because they have become aware of some of the illogicalities contained in the various classical demonstrations of the Carnot theorem.
We follow, for example, the procedure used by Mark W. Zemansky e Richard H. Dittman in their university textbook entitled “Heat and Thermodynamics”, Mc.Graw Hill Publishing Company, 1981.
The method adopted by these authors, to introduce and explain the principle of the increase of entropy, is devised as follows:
The Kelvin-Planck axiom is first presented (p. 147):
“No process is possible whose only result is to absorb heat from a source and transform this heat into work.”
An axiom attributed to Clausius is then introduced (p. 153):
“No process is possible whose only result is to transfer heat from a cold body to a warmer one.”
Pay attention to the fact that this is not exactly the axiom originally adopted by Clausius, which instead was:
“Heat cannot pass from a cold body to a warmer one without some change, connected with it, to occur at the same time.”
Below (p. 153) the authors present an argument that would show that the Kelvin-Planck axiom is equivalent to that of Clausius.
In the subsequent paragraph 7-1 (p. 158), the authors expound the concept of the “local surroundings” of a system, which are constituted by mechanisms and heat sources that interact directly with the system; below, they try to give the definition of an entity known as “the rest of the universe”, that would include:
“Other mechanical devices and heat reserves that are accessible and that could interact with the system constituted by the local surroundings of the system – or, better, the rest of the universe”.
Also in Chapter 7-1 (p. 159), the definition of a reversible phenomenon is presented:
“A reversible process is one that takes place in such a way that, at the end of the process, both the system and the local surroundings can be restored to their initial state, without producing any change in the rest of the universe.”
To be clearer, we can make this proposition explicit:
“A reversible process is one that takes place in such a way that, at the end of the process, both the system and the local surroundings can be restored to their initial state, without producing any change in any mechanical device or heat source.”
Carefully analysing the sequence of definitions above, it is clear that even for these authors the definition of reversibility arises as an immediate consequence of the axiom.
These authors, in fact, do not obtain the definition of a reversible process as a consequence of theoretical developments deriving from the axiom, but they introduce this definition before developing such theory.
Finally the authors present (p. 167) the proof by reductio ad absurdum of a theorem which, if valid, would be a preliminary to the definition of the Entropy function.
Given that the objection that is about to be raised to this demonstration is logical, it is not very important for the reader of this summary to understand all the details and meanings of the mathematical variables involved in the authors’ reasoning; it will suffice that he observes that the axiom is used twice in succession in the demonstration.
The authors would like to demonstrate the following thesis:
“Both states f1 and f2cannot be reached, starting from point i, through reversible adiabatic processes.”
To demonstrate this thesis using the principle of the excluded middle, the authors formulate the opposite hypothesis of the thesis:
“Both states f1 and f2can be reached, starting from point i, through reversible adiabatic processes.”
Then they elaborate the proof by reductio ad absurdum that ends with the observation that the advanced hypothesis leads to a condition that “… violates the Kelvin-Planck statement.”
Since, according to the authors, this is an “absurdity”, they conclude that the hypothesis put forward is impossible, so the opposite of the hypothesis must be true: the thesis must be true.
If we now analyze the sequence of these arguments, we realize that the axiom has been used twice in succession: it has been used (implicitly) a first time to define the reversible adiabatic process, and was used (explicitly) a second time to invoke the absurdity, and therefore it is not permissible to continue, in that reasoning, invoking the same axiom again to draw a subsequent deduction.
This is the first reason why the above demonstration cannot be considered satisfactory.
However, our critical analysis of the above demonstration is not yet over, because it is clear that the reasoning presented by the authors is not satisfactory for a second reason: it is incomplete.
It is true that if the hypothesis were true then the Kelvin-Planck axiom would be violated, but the authors have shown that this axiom is equivalent to CLAUSIUS’ axiom.
Therefore, the hypothesis would lead, in the final analysis, to violating CLAUSIUS’ axiom:
“The heat cannot pass from a cold body to a warmer one without some change, connected with it, to occur at the same time.”
Ultimately, the hypothesis would determine the passage of heat from a cold body to a warmer one without any compensation.
But it can be objected that the authors have not taken all the possibilities into consideration, and that their reasoning can be completed as follows:
If a quantity of heat has passed from a cold body to a warm one, it is possible to return all this heat back on its own to the cold body, by means of the natural phenomenon of heat conduction.
By inserting a “thermal resistance” between the hot body and cold body for a certain time, all the heat that had been passed from the cold body to the warm one would return back on its own to the cold body, and the situation would return exactly the same as the starting one, “without some other change, connected with it, occurring at the same time” – CLAUSIUS’ axiom would no longer be violated.
Theorists cannot say that thermal resistance cannot be introduced because this is equivalent to introducing an irreversible transformation into the system: they would use the axiom for a second time.
In fact, the axiom was used a first time to end the proof, and therefore it is not permissible to continue in that reasoning, invoking again the same axiom to derive a subsequent deduction; using the same axiom several times in a reasoning allows the tautological demonstration of any proposition.
In conclusion, it cannot be assumed that with reasoning similar to that used by the authors it is possible to demonstrate, with absolute certainty, the thesis of a fundamental theorem for the entire theory of the Second Principle of Thermodynamics – theorem that, if it were valid, would be preparatory to the proof of the existence of a function called Entropy, and to the subsequent Principle of the Increase of Entropy.
Chapter 10 – General considerations
Summarizing the above concepts, we come to the conclusion that the classical theory of the Second Principle of Thermodynamics is nothing but a well disguised tautological construction that has deceived generations of scientists.
At least one general consideration can be drawn from all the above:
Given that a dual value has been attributed to the axiom – it not only prohibits the existence of a Perpetual Motion machine of the second kind, but also declares that heat conduction is an “irreversible” transformation – then this axiom cannot be used in any proof by reductio ad absurdum of theorems relating to the Second Principle of Thermodynamics.
These demonstrations, in fact, are all substantially based on the ideation of a system in which some heat passes from a cold body to a warm one without any compensation, in order to be able to invoke the axiom to establish that such a system cannot exist.
The idea of introducing a thermal resistance into the system to make all the heat return to the cold body on its own, has so far been denied by theorists with the argument that the introduction of the thermal resistance would introduce an irreversible phenomenon into the system.
In reality, this motivation fails if the criticisms expressed above are acknowledged.
Since there are no other impediments to the introduction of a thermal resistance, the axiom remains in every circumstance an empty petition of principle, always useless in any proof by reductio ad absurdum concerning the Second Principle of Thermodynamics.
The consequences that can be drawn from these reflections are dramatic for Physics, or rather they would be if the Scientific Community manifested the intention to take note of them.
Only when this happens, one will recognize the need to abandon the classical theory concerning the Second Principle of Thermodynamics.
A void would open that could only be filled by some rationalist mathematical physicists; a brave theorist, capable of re-founding the “true” Second Principle of Thermodynamics on the basis of very different axioms.
The most important consequence for humanity would derive from the impossibility that theorists would encounter in continuing to maintain that it is impossible to build a thermal engine that works even if it does not have a heat sink (a Perpetual Motion machine of the second kind).
But the majority of the Scientific Community does not seem to be willing to admit to having sustained, for over 160 years, such a devastating physical principle for humanity, without noticing the trivial errors of Logic contained in the theory of this Principle.
We can have clear evidence of this retrograde attitude, if we note that in recent years (1988, 2000, 2004 and 2012) some scientists have devised, built, patented and published studies related to Perpetual Motion machines of the second kind, or in any case devices that highlight the error of the axiom – studies of which the Scientific Community has not been able to take note.
In reality, the Scientific Community has created the conditions on its own for not being able to immediately take note of such important scientific works.
Let us examine, in fact, the behaviour that the Scientific Community has held in occasion of the above mentioned publications, taking the 1988 publication as an example: the theoretical and experimental study that the physicist Xu Yelin conducted at the Institute of Biophysics, Chinese Academy of Sciences, Beijing – institute that published his memoir in 1988 with the title:
“A Trial and Study on Obtaining Energy From a Single Heat Reservoir at Ambient Temperature”.

Figure 10 Cover of the memoir of the physicist Xu Yelin
Link for Xu Yelin’s book:
The fact that a study with such a shocking title had been published by the Chinese Academy, should have been a guarantee for the Scientific Community, but it was not so.
The news that a Chinese scientist had invented a device able to produce electricity exploiting the environmental heat, went around the world and was reported by newspapers and newscasts.
Fortunately, I managed to obtain a copy of the publication, and I had it examined by a university professor renowned for his profound knowledge of the Second Principle of Thermodynamics, who immediately classified Xu Yelin’s study as a hoax.
Not convinced of the judgment of this distinguished professor, I wrote a book entitled “Riflessioni sulla Potenza Motrice del Calore Ambientale – e sulle machine idonee a sviluppare questa Potenza”, in which I reported, among other things, extensive excerpts from Xu Yelin’s publication.
No publishing house wanted to publish this book, based on the reports of unsigned experts, who rejected my work on the basis of laughable considerations.
In 1993 I opened a publishing activity called Astrolabium, with the sole purpose of publishing (in Italian) the book in paper format.
Astrolabium sent a free copy of the book to all university libraries in Italy.
In subsequent years, numerous copies of this book were sold to the attendees of various conferences on alternative energy to which I was invited as a speaker. Altogether, about 300 copies of the book were sold.
The American magazine “Infinite Energy” published Xu Yelin’s entire study in issue 37, Volume 7, 2001.
Many years later, I summarized the essential contents of my study on the Second Principle of Thermodynamics, including Xu Yelin’s study, expressing them in the introductory paper of the Conference “Disputes on Thermodynamics and Life”, held December 15, 2008 at “Roma-Tre” University.
Vincenzo Valenzi, the conference organizer, also promoted the publication of that report of mine (translated into English) on the CIFA-ICEF website (Comite International de Recerche et d’Etude de Facteurs de l’Ambiance).
Therefore, my aforementioned report is found (from 2011 until today) on the website: www.cifafondation.org under the button CIFA News (no. 44, Jan-Jun 2011) with the title “Reflections on the Second Principle of Thermodynamics”.
On the occasion of that conference, I distributed for free to all those present in the room a Compact Disk containing my introductory report and other support files, including Xu Yelin’s report.
In March 2013, the magazine Nexus New Times (Italian edition) published the news in the column SCIENCE NEWS (p. 49) that Philip Hardcastle had carried out an experiment very similar to that of Xu Yelin, which shows the erroneousness of KELVIN’s axiom; this experiment can be repeated by anyone, since it uses a thermionic tube that can be purchased commercially.
In March 2019 I again published as eBook on Amazon’s international platform the same book published in paper form in 1993, but this time in English, with the title: “Reflections on the Motive Power of the Environmental Heat – and on the engines suitable for producing this power”, in which is reported, among other things, the study of Xu Yelin.
In April 2019 I published another eBook in two different languages, which contains, among other things, a report on an experiment I conducted along the lines of HARDCASTLE (mentioned above), which confirmed the violation of KELVIN’s axiom.
The titles of these two books are the following: “Libro Incompiuto sull’Energia dell’Ambiente” and “Unfinished Book on the Energy of the Evironment”.
Having said that, what was the reaction of the Scientific Community to these facts concerning Xu Yelin’s memoir ?
The answer can be summarized in a few words: no reaction, with one exception represented by a memoir of the physicist Leonardo Chiatti entitled:
“Has the second Law of Thermodynamics really been violated ?”
This memory is published by Cornell University Library, and is traceable to the following web address:
https://arxiv.org/abs/physics/0702150
With this memoir, this author did not dispute the outcome of Xu Yelin’s experiment, i.e., that the electric current runs through the load resistor, but he framed the experiment in the category of phenomena in “thermal equilibrium”.
In reality in Xu Yelin’s experiment there is no thermal equilibrium, given that heat flows by conduction (due to a difference in temperature) from the environment towards the inside part of the device, since the latter cools down on its own as it produces electric current.
The fact that the electric current crosses the load resistor would not violate the axiom, according to CHIATTI, since the system would be in thermal equilibrium; the Principle would be violated, according to CHIATTI, only if the system were in Thermodynamic Equilibrium !
As is evident, CHIATTI’s interpretation does not explain where the energy that heats the load resistor comes from !
But what are the reasons why the Scientific Community has not been able to understand the message that has been pouring down on it from many quarters concerning Xu Yelin’s experiment ?
On the occasion, the Scientific Community behaved like Peripatetic philosophers, who in the face of experiments that showed that Nature did not behave according to the Aristotelian philosophy, rejected the value of experience.
These facts suggest that the hope that the Scientific Community will spontaneously take note of what is reported in this summary is in vain; although it was read by some of them who shared the criticisms expressed here, they would not externalize them to other colleagues pretending that this document does not exist.
It has been said above that it was the Scientific Community that created the conditions for not being able to immediately take note of scientific works that challenge established theories.
In fact, the Scientific Community got into the habit of not reading, but above all not taking seriously, articles and memoirs not subjected to the Peer-Review process, just like the one you are reading !
This attitude delays for years, and in some cases for centuries, the benefits that such works can bring to science and mankind.
Chapter 11- The Peer-review Process
The scientific Editors that are appreciated by scientists are those who submit the works received with publication requests to the Peer-Review Process.
This process was established to prevent the publication of works containing errors committed by the authors, or articles containing scientific frauds.
The task of evaluating scientific works is entrusted to some Auditors, who are members of the Scientific Community that are considered very experienced and appreciated in the scientific field related to the works to be judged.
Therefore, they are considered “peer” (from the scientific point of view) compared to the authors hoping to obtain the publication of a work. It is for this reason that such process of selecting the articles worthy of being published is called the “Peer-Review Process”.
Therefore, these great experts in a field of science, in which they have become so famous as to be paid as auditors, must determine whether a new scientific work can be published or not.
However, a debate has been going on for some time about the effectiveness of the peer review process, as many scientists are not at all satisfied with how this system works.
A remarkable article on this topic has recently been published by the magazine Nexus New Times (English edition, Jun-July 2018, Vol. 25 N. 4).
The article entitled: “Failure of Peer-Review: Especially in Medicine” is by Brendan D. Murphy
MURPHY’s article reports a series of incredible facts, supported by 25 bibliographic citations, concerning the malfunctioning of the Peer-Review process currently in force. We are only reporting one, by way of example: (Richard Horton, “Genetically modified food; Consternation, confusion and crack-up”, guest editorial in the Magazine The Medical Journal of Australia, 172 (4), 2000).
MURPHY’s quotation concerning Richard Horton, publisher of The Lancet, is the following:
“The mistake, certainly, is to have thought that the peer-review process is nothing but a rough tool to discover the acceptability – not the validity, of a new discovery… We present the peer-review process to the public as an almost sacred process, which helps make science our goal oracle of truth. But we know that this system is biased, unfair, unreliable, incomplete, easy, often offensive, normally ignorant, sometimes insane, and frequently wrong.”
In the face of all this it is legitimate to ask: what happens if the new work that needs to be evaluated goes to upset the scientific sector in which these Reviewers excel ?
It happens that these great experts come into a conflict of interest: if the new and shocking work were published, they would risk losing the status, and with it the paid positions of Scientific Auditors.
Add to this the fact that the Auditors can be subject to pressure aimed at influencing the science in a certain direction, and that the reviewer’s judgment is protected by anonymity, it can be understood how it is highly probable that this new and unsettling work will not be considered valid and will not be published by the prestigious Editor; even if it was published by unqualified publishers, the Scientific Community would not take it into account, given the habit of scientists to take seriously only what has been submitted to the peer-review process.
The new shocking work would thus be considered non-scientific by the Scientific Community, and therefore all the more reason so by everyone, like incorrect or fraudulent works !
With these premises, you who are reading this summary, do you think by chance that it could be published by a qualified Editor ?
If you think so, then you are sure that the Scientific Community will read, sooner or later, a work written by the author on the erroneousness of the Second Principle of Thermodynamics, and will readily change opinion, with all the positive consequences that this will entail for everyone.
If, on the other hand, you do not believe that all this can happen, then you could do something so that the positive consequences that have been described previously occur.
Chapter 12 – But what could you do ?
At the beginning of this summary it was said that a Community of Citizens intent on combating global warming should take note of the erroneousness of the current version of the Second Principle of Thermodynamics.
But then, once the above arguments had convinced groups of citizens of this mistake, what could they do to counteract global warming ?
There is practically only one way to achieve this result: to seek the system of convincing the Scientific Community to take note of the erroneousness of the current version of the Second Principle of Thermodynamics; a difficult task (but not impossible), given that almost all men of science are firmly convinced of the validity of such Principle.
What a Community of Citizens could do depends on the level and role that its members hold in the Society. Some communities may have members that are part of policy, science, or are members of the second level or university school (teachers and students), and so on.
For instance, high school or university students could require their physics teachers to repeat, in the institute labs, the experiment described above, to verify the non-generality of KELVIN’s axiom.
In any case, any initiative should be aimed at obtaining a single result:
Create the conditions for a State Authority to make sure that one or more members of the Scientific Community pronounce on the criticisms of the Second Principle of Thermodynamics expressed by the author of this summary, by means of a signed report that could be used in any institutional venue.
This would start a debate that could lead the Scientific Community to the decision to do what in 160 years has never been done: submit the Second Principle of Thermodynamics to a crucial experiment: an Experimentum Crucis.
In fact, it is true that a Second Principle of Thermodynamics exists, but that one shared by the Scientific Community today is not the right one !